‘This is absolutely transformative’: ‘Heart in a Box’ saves Minnesota man’s life, could change heart transplants
[anvplayer video=”5007885″ station=”998122″]
Currently, 3,511 Americans are waiting to receive a heart transplant. That includes 122 Minnesotans.
Minneapolis Heart Institute Foundation (MHIF) is one of only seven clinical sites in the country participating in a new research study to evaluate a piece of new technology that has the potential to change the way heart transplants are done.
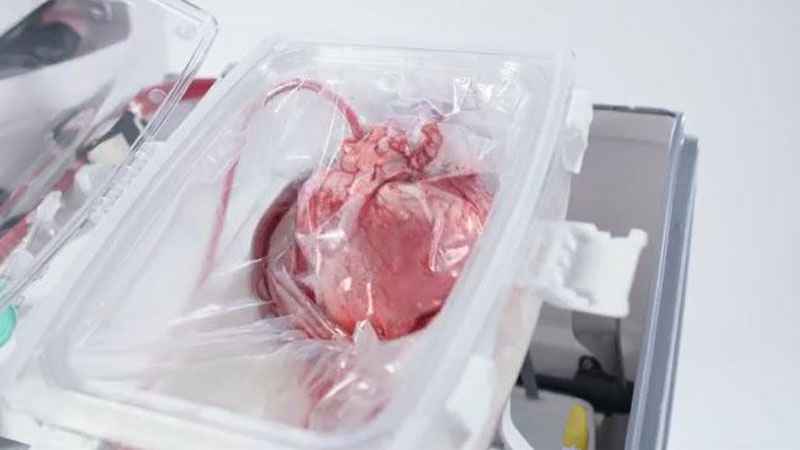
It’s called ‘Heart in a Box’ and is made by TransMedics. It’s a portable, warm perfusion and monitoring system designed to keep a donor heart at a human-like, metabolically active state. By monitoring key parameters of the functioning heart, physicians may use their medical judgment to assess a potentially suitable heart’s condition and viability.
"It allows us to go greater distances," Dr. Benjamin Sun, MHIF surgical director of heart failure, said. "And secondly, it allows us to look at hearts that perhaps were not as ideal as we would like them to be."
Wisconsin woman meets family of Minnesota teen whose heart she received
[KSTP]
Traditional heart donations come from a donor who is declared brain dead but remains on life support. This is called donation after brain death, or DBD.
An alternative number of donations are performed in patients who have had catastrophic brain injuries but are not brain dead. These patients are allowed to pass after their hearts stops beating. Organ procurement for kidneys, lungs and livers have been successfully utilized from these donors. This is called donation after circulatory death, or DCD. Unfortunately, a heart cannot be procured with traditional methods from these donors. They can be used with Heart in a Box.
The "beating heart" transplant technology reanimates and resuscitates a DCD donor’s heart that has stopped beating. The support system allows the heart to recover and continually beat in its self-contained module.
"For us, this is absolutely transformative," Dr. Sun said. "We’re very excited that, as we get more data and if the results continue to be as we’re seeing them here, that this has the potential to totally change how we approach organ donation."
The Organ Care System (OCS) continually monitors the heart while in transport.
"From this device, we can actually assess how this organ is doing and how the organ is doing over time," said Dr. Karol Mudy, an MHIF cardiothoracic surgeon. "It really allows us to learn about the organ that is en route to the recipient site and make a final call, a final decision at the recipient site, whether we really believe this organ is suitable for transplantation."
And a heart can last up to nine hours, which greatly increases the distance a donor heart can come from, especially if the Heart in a Box is on a plane.
"The process involves us doing a normal procurement," said Dr. Sammy Shukrallah, an MHIF organ recovery surgeon. "We take the heart out. And then we put it onto this machine and start feeding blood through the heart. The heart, at that point, is what we call reanimating; we turn it on. And then we begin to pace it. The next thing we begin to do is follow markers. This heart will produce certain markers that we’re looking at, lactates specifically. These are the exciting moments for all of us really, to see what those markers are doing as this heart gets reanimated and/or turned on."
Fifty-seven-year-old Kevin Manion had a heart transplant at MHIF using a DCD donor heart and Heart in a Box technology on Jan. 30.
"I feel good," he said. "I feel like I’m making a little bit of progress every day and finding a new routine."
Manion didn’t hesitate to be part of the Minneapolis Heart Foundation Institute research.
"You know, I’m kind of at a point in life where you want to make a contribution of some level, and this seemed like a real easy thing for me to do and maybe help some other people along the way."
The TransMedic Heart in a Box is already commercially available in Europe and Australia, and wait times for hearts have been greatly reduced. It could receive U.S. Food and Drug Administration approval for use in the U.S. in a few months.